
Hcn Lewis Structure Bonds Draw Easy
The Lewis dot diagram for HCN is as follows: The hydrogen atom is represented by a single dot, the carbon atom by four dots (arranged horizontally or vertically), and the nitrogen atom by five dots (arranged in a cross shape). These dots represent the valence electrons of each atom. The hydrogen atom shares its electron with carbon, and carbon.
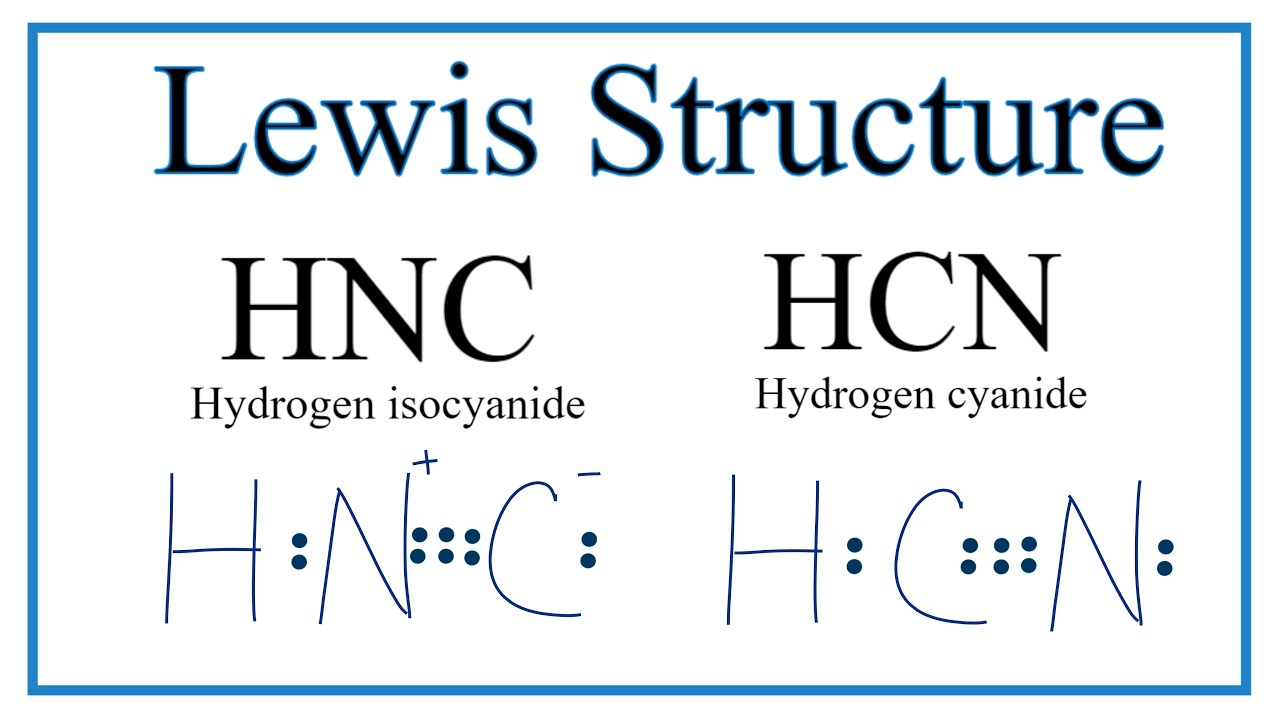
Hcn Lewis Structure Bonds Draw Easy
This video shows you how to draw Lewis Dot structure in 5 easy steps.1. Count total valence electrons for the molecule.2. Put the least electronegative atom.

HCN Lewis Structure How to Draw the Dot Structure II lSCIENCE ll NCERT ll Rohit Sir YouTube
Step-1: HCN Lewis dot Structure by counting valence electrons on the carbon and nitrogen atom. To calculate the valence electron of each atom in HCN, look for its periodic group from the periodic table. The carbon, nitrogen, and hydrogen group families, which are the 17th and 1st groups in the periodic table, are both made up of carbon.

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw Lewis Structure of HCN
HCN, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. It is linear molecule with a triple bond between C and N atom and has bond angle of 180 degrees.. The Valence Bond thoery simply explains the bond formation just like lewis dot structure, but instead it explains the bonding in terms of covalent bond by.

HCN Lewis Structure HCNLewisStructure Lewis Dot Structure for HCN YouTube
Drawing the Lewis Structure for HCN. Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you'll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. Be sure that you don't use more than the ten valence electrons available.

So far, we’ve used 8 of the HCN Lewis structure’s total 8 outermost valence shell electrons. One
HCN Lewis dot structure consist of 3 elements as shown in the formula. Due to electronegativity difference carbon is the central atom which shares its 1 electron with hydrogen and 3 electrons with nitrogen to fulfill the stability criteria. This leads to formation of carbon forming single covalent bond with hydrogen and triple covalent bond.
[Solved] Draw the Lewis structure for it as well. 3. Hydrogen cyanide (HCN)... Course Hero
The Lewis Structure (Lewis Dot Diagram) for HCN.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out.

lewis dot diagram for hcn Wiring Diagram
For the HCN Lewis structure, calculate the total number of valence electrons for the HCN molecule.

Molecular Geometry, Lewis Structure, and Bond Angle of HCN
Steps for Writing Lewis Structures. Calculate the sum of the valence electrons in the molecule. 1 C atom = 1 × 4 = 4 valence e -. 1 O atom = 1 × 6 = 6 valence e -. 2 Cl atoms = 2 × 7 = 14 valence e -. sum of valence e - = 24 valence e -. Construct a skeleton structure for the molecule. C is the central atom since it makes the most.

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
HCN is a highly toxic substance that has a bitter almond-like smell. There is one bond between H and C and three bonds between C and nitrogen. There is one lone pair of. electrons on the nitrogen atom. The compound has sp hybridization. The molecular geometry of HCN is linear. The compound is polar in nature.
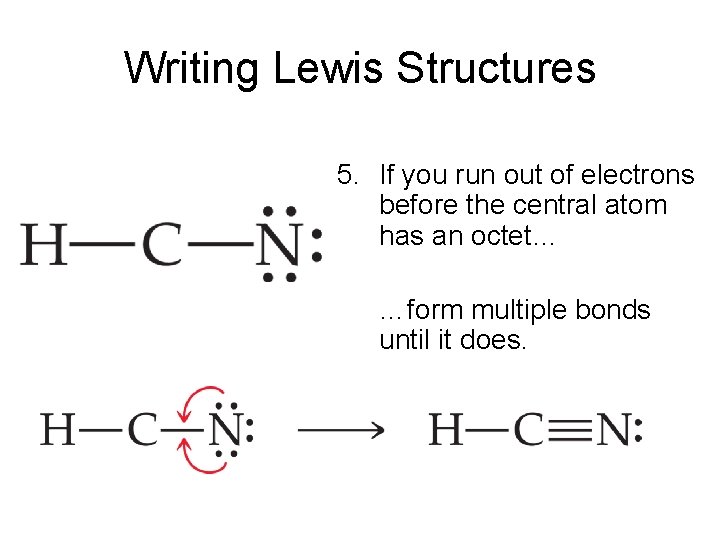
Hcn Lewis Structure Bonds Draw Easy
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN Lewis Dot Structure YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is HCN. In order to draw the lewis structure of HCN, first of all you have to find the total number of valence electrons present in the HCN molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
10+ Lewis Dot Structure For Hcn Robhosking Diagram
Steps. Use these steps to correctly draw the HCN Lewis structure: #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required #4 Convert lone pairs of the atoms, and minimize formal charges #5 Repeat step 4 if needed, until all charges are minimized, to get a stable Lewis structure

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Techiescientist
Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule .

Lewis Dot Diagram Of Hcn
Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table.
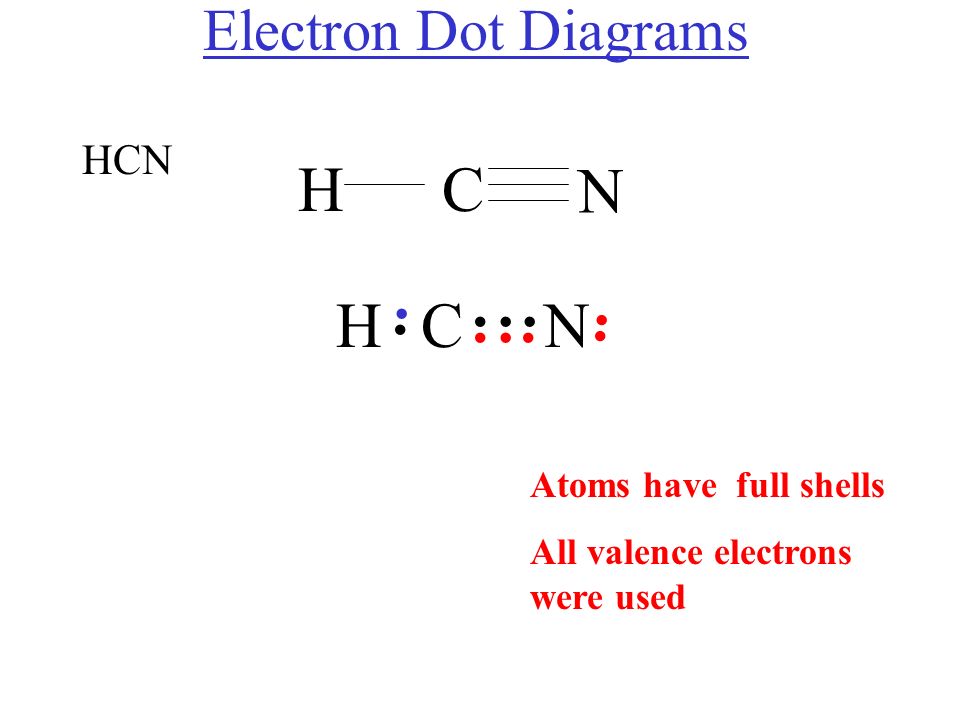
Diagrama De Lewis Hcn Estudiar
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share.